Function of Salt Bridge
UV-B photoreception causes rapid. It basically helps in preventing the accumulation of positive.
Salt Bridge Definition Function Types Preparation Galvanic Cells
It basically helps in preventing the.

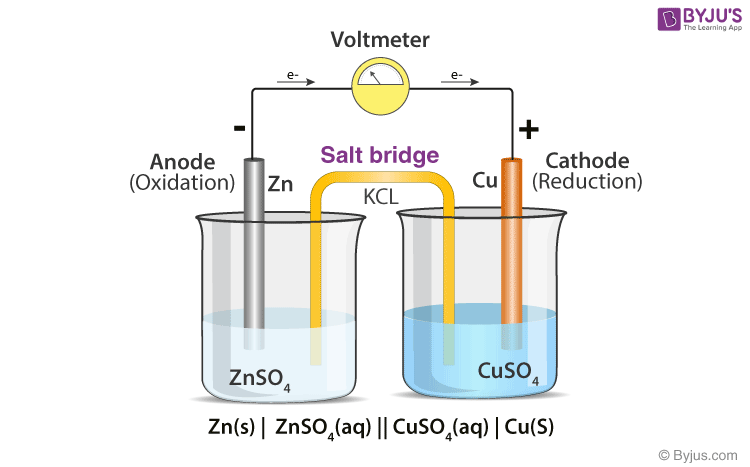
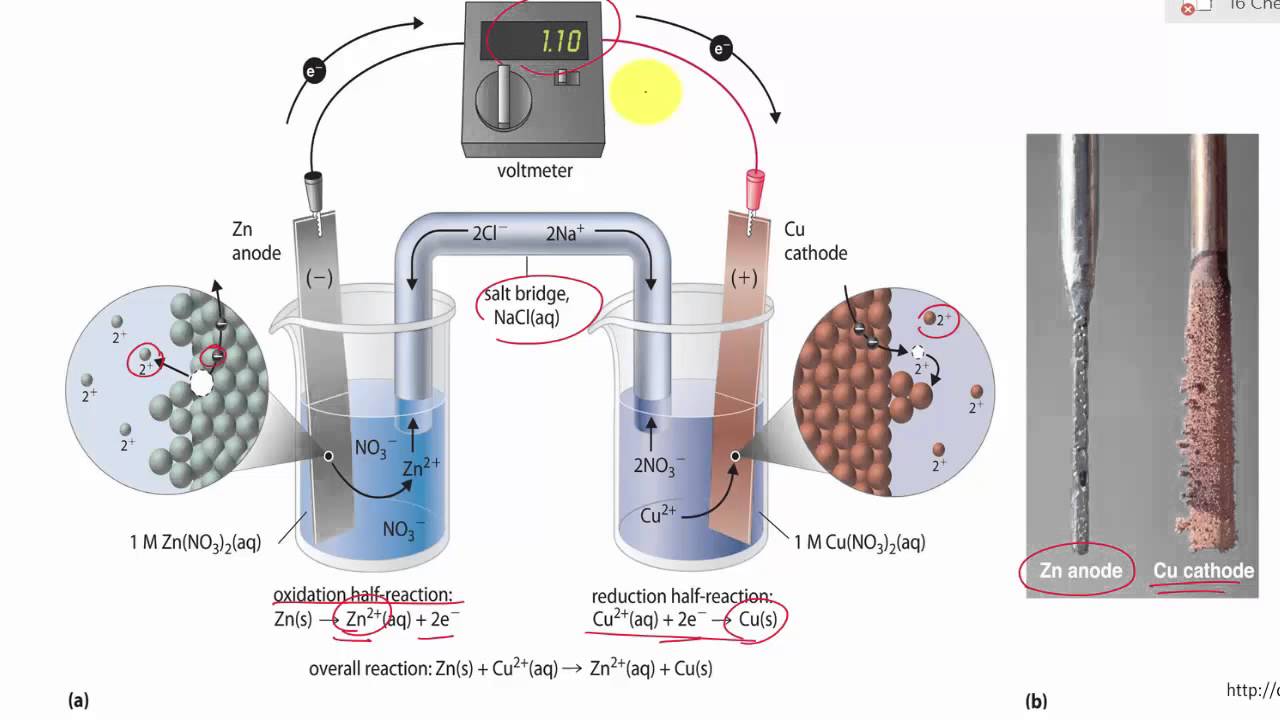
. In electrochemistry a salt bridge or ion bridge is a laboratory device used to connect the oxidation and reduction half-cells of a galvanic cell a type of electrochemical cell. The main function of a salt bridge is to maintain the electrical neutrality of both electrolytic solutions within the internal circuit. Hi We use a salt bridge in a Galvanic cell to maintain electron neutrality by transferring anions to the anode side and cations to the cathode.
Salt bridge complete the electrical circuit. Also it helps to prevent the cell from taking its reaction to. Prevention of the diffusion or mixing of the two electrolyte solutions while maintaining the constant flow of electrons.
The function of a salt bridge in electrochemical cell is to keep electrical neutrality in the solutions by providing an electrical contact between both sides of the galvanic cell. A salt bridges major function is to help maintain the electrical neutrality within the internal circuit. The salt bridge connects the two solutions of the half cells and their electrodes are.
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators. UV RESISTANCE LOCUS8 UVR8 is a photoreceptor for ultraviolet-B UV-B light that initiates photomorphogenic responses in plants. The function of salt bridge in electrochemistry.
3 3Salt bridge Wikipedia. A salt bridge Ion bridge is a device that connects oxidation and reduction half cells of a galvanic cell. A maintain electrical neutrality of both half cells B increase the cell potential at the positive electrode C decrease the cell potential at the negative electrode D.
A salt bridge acts as an electrical contact between the two half cells. 2 2Salt Bridge Definition Function Types Preparation Galvanic Cells. The functions of a salt bridge are.
The two half-cells of a galvanic cell remain neutral as a result. It prevents the mechanical flow of solution but it provides a free. 1 1Why is it important to use a salt bridge in a voltaic cell.
What is the function of a salt-bridge in an electrochemical cell. Electrons move from the anode to the cathode. The functions of the salt bridge are.
The main function of a salt bridge is to maintain the electrical neutrality of both electrolytic solutions within the internal circuit. The salt bridge allows cations to move in the galvanic cell. The function of a salt bridge is to.
The important functions of the salt bridge are.

Salt Bridge Definition Function Types Preparation Galvanic Cells

Kac32 17 Electrochemistry The Role Of The Salt Bridge Youtube
Describe The Function Of A Salt Bridge Youtube

Function Of Salt Bridge Salt Bridge E Learn Foundation Youtube
No comments for "Function of Salt Bridge"
Post a Comment